The Untoward Side Effects of Interferon Therapy Correlate Well with the Spectrum of Symptoms that Make up the Down Syndrome
There is an ongoing effort to identify individual genes on Chromosome 21 that may independently each subserve one of the many diverse symptoms that constitute the Down syndrome. In contrast, the interferon system points to a multigene, multilocus, system distributed across Chromosome 21 that can explain each of the individual symptoms of the Down syndrome as a consequence of the concerted action of multiple genes which share in common a role in interferon action. To date, at least ten such genes have been identified on Chromosome 21. This number is likely to grow as new genes are identified, and their role in interferon action is uncovered. The recently discovered human SIM2 gene is presented here as an example. Its Intron 3 sequence reveals a clustering of interferon response elements that suggests an unexpected role for this gene in interferon action. We also present here a comparative analysis of the complex of untoward side effects of interferon therapy that reveals a striking similarity to the spectrum of symptoms that make up the Down syndrome. Taken together, these gene mapping and clinical observations suggest the possibility that the diverse symptoms of the Down syndrome could have a surprisingly non-diverse underlying biochemistry.
Maroun, Heffernan, and Hallam . (1998) The Untoward Side Effects of Interferon Therapy Correlate Well with the Spectrum of Symptoms that Make up the Down Syndrome. Down Syndrome Research and Practice, 5(3), 143-147. doi:10.3104/hypotheses.91
* From a paper presented at the 6th World Congress on Down Syndrome, Madrid, Spain, October 1997.
Introduction
It has been known for many years that cells from individuals with Down syndrome are excessively sensitive to interferon effects (Tan et al., 1974). Two observations suggest that this interferon hypersensitivity could underlie many Down syndrome pathologies:
Numerous genes involved in interferon action are located in the region of human chromosome 21 responsible for most of the Down syndrome pathologies.
The "distal" half of chromosome 21 contains a large cluster of genes that direct the synthesis of interferon receptors and receptor components. These genes control sensitivity to each of the various types of interferons. In addition, the same "Down Syndrome Region" (DSR) contains genes whose activities are controlled by the interferons and at least one gene that is involved in this control mechanism (ETS-2, a "transcription factor"). These genes, along with their potential role in brain function, have been described previously (Maroun, 1996).
Other genes mapped to Chromosome 21 and thought to be important in brain function are also candidate interferon responsive genes. For example, we have now found an unusual cluster of interferon response elements in Intron 3 of the human SIM2 gene (Figure 1).

Figure 1. Interferon response elements in Intron 3 of the Human Chromosome 21 SIM2 Gene.
The presence on Chromosome 21 of genes that are targets of interferon action provides an attractive explanation of the many-fold increase in interferon sensitivity seen in cells from people with Down syndrome in spite of the observation of only a fifty percent increase in interferon receptor numbers (Epstein, 1986).
A clear correlation is observed for many of the pathologies seen in a person with Down syndrome with the side effects seen in patients undergoing interferon therapy.
One of the most compelling arguments for the involvement of interferon action in the Down syndrome is based on a remarkable similarity of the spectrum of symptoms seen in Down syndrome and with those seen in patients who have been treated with interferon. Although the effects of interferon on growth were predictable given interferon's anticellular effects, the brain, heart and immune system function effects are surprising in their similarity to the pathology seen in the Down syndrome. It is possible that interferon's ability to slow cell growth may subserve each of these pathologies at their most fundamental level.
This comparison is presented in tabular form in Table 1 along with pertinent references.
| Down Syndrome Pathologies | Interferon Side Effects |
|---|---|
| Learning Difficulties (Carr, 1985) | Neurotoxicity (Mattson et al., 1983), Memory Loss (Iivanainen et al., 1985) |
| Small frontal lobes (Wisniewski et.al., 1985) | Frontal lobe encephalopathy (Mattson et al., 1984) |
| Heart Pathology (Sei et al., 1995) | Cardiotoxicity (Sonnenblick and Rosin, 1991) Arrhythmia (Martino et al., 1987) |
| Leukopenia (Cossarizza et al., 1990) | Leukopenia (Quesada et al., 1986) |
| Autoimmune disease (Levo, 1977) | Autoimmune disease (Burman et al., 1985) |
| Hypothyroidism (Mitchell et al., 1994) | Hypothyroidism (Fentiman et al., 1985) |
| Hearing Loss (Roizen et al., 1992) | Deafness (Kanda et al., 1995) |
| Short Stature (Piro et al., 1990) | Growth Inhibition (Kikawa et al., 1995) |
Anti-interferons can reverse or prevent the untoward effects of the interferons.
Because interferons can do so many apparently unrelated things, it has been traditional to demonstrate that a newly found biological activity was indeed due to interferon by reversing or preventing the effect of added interferon by simultaneously adding anti-interferon (usually an antibody) to the culture media. In all cases where the effect was due to the added interferon, the effect was, as would be expected, reversed or prevented by the anti-interferon treatment. This ability of an anti-interferon to reverse or prevent interferon actions has been extended to include "in vivo" whole animal
observations including the extension of these observations to the animal model for Down syndrome (Maroun, 1995). Injection of anti-interferon immunoglobins into mothers carrying trisomy 16 mouse fetuses resulted in significant return-to-normal values for fetal growth, eye closure, and back curvature (Maroun, 1995). In contrast to the unpredictable growth of the untreated trisomy fetuses, the growth of the treated trisomy fetuses was essentially keeping pace with that of their euploid littermates (Figure 2).
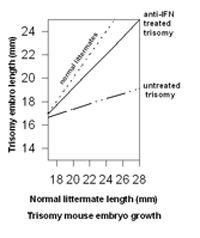
Figure 2. Improvement in the growth of trisomic mouse embryos treated with anti-IFN antibodies. Modified from Maroun, 1995.
In these experiments the reduction in interferon bioactivity was observed to be well tolerated. This is consistent with what is observed with "Knockout" mice whose genes for interferon receptors have been rendered inactive ( Hwang et al., 1995 ). The same is true in humans who carry a mutation in their interferon receptor genes ( Newport et al., 1996 ) ( Jouanguy et al., 1996 ). Patients who have circulating anti-interferon antibodies as a consequence of interferon therapy show the expected decrease in the efficacy of further interferon treatment but no other untoward effects have yet been observed (Quesada et al., 1986). Taken together, the observations discussed here suggest that the in vivo neutralization of interferon in individuals with Down syndrome could be both safe and potentially beneficial.
Acknowledgements
This project was supported in part by the American Heart Association and the Excellence in Academic Medicine program of Southern Illinois University School of Medicine.
Correspondence
L.E. Maroun, Ph.D. Southern Illinois University School of Medicine. Dept. of Medical Microbiology/Immunology. P.O. Box 19230, Springfield, Illinois USA 62794-1220. (Tel: (217) 785-2181, Fax: (217) 524-3227, E-mail: lmaroun@siumed.edu)
References
- Burman, P., Karlsson, F.A. & O Berg, K. (1985). Autoimmune thyroid disease in interferon-treated patients. Lancet, 2 (8446), 100-101.
- Carr, J. (1985). The Development of Intelligence. In D. Lane and B. Stratford (Eds.), Current Approaches to Down's Syndrome, pp. 167-186. London: Holt, Rinehart & Winston, as presented by Wishart, J.G. (1995). In Etiology and Pathogenesis of Down Syndrome, pp. 57-91. Wiley-Liss, Inc.
- Cossarizza, A., Monti, D., Montagnani, G., Ortolani, C., Masi, M., Zannotti, M. & Franceschi, C. (1990) . Precocious Aging of the Immune System in Down Syndrome: Alteration of B Lymphocytes, T-Lymphocyte Subsets, and Cells with Natural Killer Markers. American Journal of Medical Genetics Supplement, 7, 213-218.
- Epstein, C.J. (1986). Trisomy 21 and the Nervous System: From Cause to Cure. In C.J. Epstein (Ed.), The Neurobiology of Down Syndrome, pp. 1-16. New York: Raven Press.
- Fentiman, I.S., Thomas, B.S., Balkwill, F.R., Rubens, R.D. & Hayward, J.L. (1985) . Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet, 1 (8438), 1166.
- Hwang, S., Hertzog, P., Holland, K., Sumarsono, S., Tymms, M., Hamilton, J., Witty, G., Bertoncello, I. & Kola, I. (1995) . A null mutation in the gene encoding a type I interferon receptor component eliminates antiproliferative and antiviral responses to interferons a and b and alters macrophage responses. Procedures of the National Academy of Science, 92, 11284-11288.
- Iivanainen, M., Laaksonen, R., Niemi, M.L., Farkkila, M., Bergstrom, L., Mattson, K., Niiranen, A. & Cantell, K. (1985) . Memory and psychomotor impairment following high-dose interferon treatment in amyotrophic lateral sclerosis. Acta Neurologica Scandinavica, 72(5), 475-480.
- Jouanguy, E., Altare, F., Lamhamedi, S., Revy, P., Emile, J-F., Newport, M., Levin, M., Blanche, S., Seboun, E., Fischer, A. & Casanova, J-L. (1996). Interferon-(-receptor Deficiency in an Infant with Fatal Bacille Calmette-Guerin Infection. The New England Journal of Medicine, 335(26), 1956-1961.
- Kalvakolanu, D.V. & Bordon, E.C. (1996). An Overview of the Interferon System: Signal Transduction and Mechanisms of Action. Cancer Investigation, 14(1), 25-53.
- Kanda, Y., Shigeno, K., Matsuo, H., Yano, M., Yamada, N. & Kumagami, H. (1995) . Interferon-Induced Sudden Hearing Loss. Audiology, 34, 98-102.
- Kikawa, K., Nishida, K., Tanizawa, A., Shigematsu, Y., Nakai, A., Sudo, M. & Matsuyama, T. (1995) . Growth retardation as a long-term side-effect of alpha-interferon therapy. European Journal of Pediatrics, 154(7), 591-592.
- Levo, Y. (1977). Down syndrome and autoimmunity. Am. J. Med. Sci., 273, 95-99.
- Maroun, L.E. (1995). Anti-Interferon Immunoglobulins Can Improve the Trisomy 16 Mouse Phenotype. Teratology, 51, 329-335.
- Maroun, L.E. (1996). Interferon Action and Chromosome 21 Trisomy (Down Syndrome):15 years later. Journal of Theoretical Biology, 86, 603-606.
- Martino, S., Ratanatharathorn, V., Karanes, C., Samal, B.A., Ho-Sohon, Y. & Rudnick, S.A. (1987) . Reversible arrhythmias observed in patients treated with recombinant alpha-2 interferon. Journal of Cancer Research and Clinical Oncology, 113, 376-378.
- Mattson, K., Niiranen, A., Iivanainen, M., Farkkila, M., Bergstrom, L., Holsti, L.R., Kauppinen, H.L. & Cantell, K. (1983 ). Neurotoxicity of interferon. Cancer Treatment Reports, 67, 958-961.
- Mattson, K., Niiranen, A., Laaksonen, R. & Cantell, K. (1984). Psychometric monitoring of interferon neurotoxicity. Lancet, 1(8371), 275-276.
- Mitchell, C., Blachford, J., Carlyle, M. & Clarson, C. (1994). Hypothyroidism in patients with Down syndrome. Archives of Pediatrics & Adolescent Medicine, 148(4), 441-442.
- Newport, M., Huxley, C., Huston, S., Hawrylowicz, C., Oostra, B., Williamson, R. & Levin, M. (1996) . A Mutation in the Interferon-(-receptor Gene and Susceptibility to Mycobacterial Infection. The New England Journal of Medicine, 335(26), 1941-1949.
- Piro, E., Pennino, C., Cammarata, M., Corsello, G., Grenci, A., Lo Giudice, C., Morobito, M., Piccione, M. & Giuffre, L. (1990). Growth Charts of Down Syndrome in Sicily: Evaluation of 382 Children 0 - 14 Years of Age. American Journal of Medical Genetics Supplement, 7, 66-70.
- Quesada, J., Talpaz, M., Rios, A., Kurzrock, R. & Gutterman, J. (1986). Clinical Toxicity of Interferons in Cancer Patients: A review. Journal of Clinical Oncolog y, 4(2), 234-243.
- Roizen, N., Wolters, C., Nicol, T. & Blondis, T. (1993). Hearing loss in children with Down syndrome. Journal of Pediatrics, 123, S9-12.
- Sei, H., Enai, T., Chang, H-Y & Morita, Y. (1995). Heart Rate Variability During Sleep in Down's Syndrome. Physiology and Behavior, 58(6), 1273-1276.
- Sonnenblick, M. & Rosin, A. (1991). Cardiotoxicity of Interferon - A Review of 44 Cases. Chest, 99, 557-561.
- Tan, Y.H., Schneider, E.L., Tischfield, J., Epstein, C.J. & Ruddle, F.H. (1974) . Human Chromosome 21 Dosage: Effect on the Expression of the Interferon Induced Antiviral State. Science, 186(4158), 61-63.
- Wisniewski, K.E., Wisniewski, H.M., & Wen, G.Y. (1985). Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Annuals of Neurology, 17, 278-282.

