Oxidative stress in Portuguese children with Down syndrome
Background - Individuals with Down syndrome have an accelerated process of ageing which is thought to be associated with oxidative stress. Aim - Since Zn/Cu superoxide dismutase is increased by about 50% in children with Down syndrome, glutathione and other less known antioxidant mechanisms were studied to determine whether there were changes in reactive oxygen species. Methods - Plasma reduced and oxidised glutathione and red blood cells enzymes including acid phosphatase, methemoglobin reductase and transmembrane reductase were evaluated in Portuguese children with Down syndrome and their siblings, who were used as a control group. Results - No significant differences were found between the study and control groups. A negative correlation was noted between total glutathione and acid phosphatase in the siblings without Down syndrome, but not in the children with Down syndrome. Conclusion - Although it is claimed that the production of hydrogen peroxide is enhanced in children with Down syndrome, their antioxidant mechanisms do not seem to be significantly different compared with their siblings. This may result in an excess of reactive oxygen species that could help to explain accelerated ageing in children with Down syndrome. Further studies will be needed to shed light on these mechanisms.
Pinto, M, Neves, J, Palha, M, and Bicho, M. (2002) Oxidative stress in Portuguese children with Down syndrome. Down Syndrome Research and Practice, 8(2), 79-82. doi:10.3104/reports.134
Introduction
Individuals with Down syndrome seem to have an accelerated process of ageing (Carmeliet, David & Cassiman, 1991; Prasher, 1993; Pueschel, 1990), evidenced by early onset of cataracts and the risk of developing Alzheimer's disease. Ageing is believed to be a condition associated with free radical production (Busciglio & Yanker, 1995; Crastes de Paulet, 1990; Rubin, Gatchalian, Rimon & Brooks, 1994).
During oxidative stress, harmful reactive oxygen species are generated (Crastes de Paulet, 1990). Superoxide radicals are converted to hydrogen peroxide by Zn/Cu superoxide dismutase (SOD) (De-Haan, Cristiano, Innello & Kola, 1995; Gerli et al., 1990). Thereafter, several enzyme systems, including glutathione peroxidase (GPX) and catalase (CAT), independently convert it to water (De-Haan et al., 1995; Gerli et al., 1990), see Figure 1.
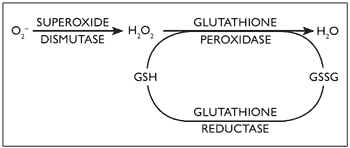
Figure 1. Glutathione peroxidase (GPX) role in oxidative stress and its interaction with superoxide dismutase (SOD)
The SOD gene locus resides on chromosome 21 and as a consequence of gene dosage excess, SOD activity has been shown to be increased by about 50% in all tissues of patients with Down syndrome (Brooksbank & Balzas, 1983; Ceballos-Picot, 1993; De La Torre et al., 1996). An imbalance between SOD activity and GPX was proposed as an important determinant of molecular ageing, since the resultant hydroxyl radicals which are highly reactive could cause damage to macromolecules such as DNA, protein and lipids (Bar-Peled, Korkotian, Segal & Groner, 1996; De-Haan, et al., 1996). These free radicals are normally neutralised by free radical scavengers and other antioxidant enzyme systems (Gerli et al., 1990).
Glutathione (GSH) represents a well-known major physiological mechanism of response to oxidative stress, interacting with SOD, as shown in Figure 1 (De-Haan et al., 1996; Ishikawa & Sies, 1989). Acid phosphatase (ACP1) is a tyrosine phosphatase protein, and is affected by reactive oxygen species. It has a role as flavin mononucleotide (FMN) phosphatase and subsequently interferes with glutathione reductase activity (Chiarugi, et al., 1996; Fuchs, Shekels & Bernlohr, 1992; Gerli, et al., 1990; Magenis, et al., 1975; Mohreweiser & Novotny, 1982).
Abbreviations
ACP1 - acid phosphatase
CAT - catalase
FMN - flavin mononucleotide
GPX - glutathione peroxidase
GSH - plasma reduced glutathione
GSSG - oxidised glutathione
MHR - methemoglobin reductase
SOD - Zn/Cu superoxide dismutase
TMR - transmembrane reductase of ferricyanide
Methemoglobin reductase (MHR) is an erythrocyte cytosolic system responsible for reactivating haemoglobin's ability to transport oxygen after it has been converted into methemoglobin by reactive oxygen species (Board & Pidcock, 1981; Hatherill, Till & Ward, 1991). Transmembrane reductase of ferricyanide (TMR) expresses the existence of an erythrocyte transmembrane redox system responsible for, among others, recycling antioxidants (Hatherill et al., 1991; May, Qu & Morrow, 1996; Orringer & Roer, 1979).
Our purpose was to study some of these systems including plasma GSH and red cell ACP1, MHR and TMR activities in a sample of Portuguese children with Down syndrome. Considering the possibility of intervening factors from the environment, we decided to study the siblings of children with Down syndrome as a control group. We believe that by doing this the two groups would have similar environmental exposure, nutrition and social, cultural and genetic background.
Population and methods
During a visit to the Child Development Centre of the Paediatric Department of the Santa Maria University Hospital of Lisbon, the aims of the study were explained to the parents of children with Down syndrome. They were then asked to give consent for their children to be enrolled in our study and to bring their other children on the next visit.
All children attending the clinic during the time period of the study were invited to take part. All but three families with non-Down syndrome siblings agreed to participate. The three families not participating were from a distant part of the country. Two groups were defined: 60 children with Down syndrome and 29 siblings without Down syndrome. The mean age of the children with Down syndrome was 3.6 years (SD 3.33; range 0.5 to 12 years), and in the population of siblings without Down syndrome the mean age was 7.3 years (SD 4.48; range 1 to 17 years). There was a significant age difference between the two groups, t(82) = -4.153, p = .0001. The sex distribution in the population with Down syndrome was 43% females and 57% males; in the sibling group the distribution was 51% and 48%, respectively.
Blood samples of children with Down syndrome and their siblings were collected by venipuncture and were analysed blind by the Genetic Laboratory of the Faculty of Medicine of Lisbon, between the 1st of April of 1995 and the 1st of April of 1996. Each of the tests was performed by an experienced technician, replicated three times and the mean was used as the test result.
Plasma GSH/GSSG was measured by an adapted fluorimetric assay of Hissin and Hilf (1976) and expressed as μg/g protein. Red blood cell TMR was performed using a method modified by Orringer and Roer (1979), expressed as mmol/l cell/h. MHR in the same cells was evaluated by a spectrophotometric assay described by Board and Pidcock (1981), expressed as μmol/g Hb/min, and ACP1 activity was measured by the method of Magenis, et al. (1975), expressed as μmol/g Hb/min.
Data are presented as mean (standard error of the mean) and analysed with the paired t Student test and significance was accepted for p value < 0.05. The statistical software "Primer of Biostatistics, version 3.02" was used.
Results
Plasma glutathione levels (reduced and oxidised forms) are presented in Table 1. The siblings without Down syndrome had higher concentrations of GSH (reduced form), GSSG (oxidised form) and total GSH, but the difference was not statistically significant.
| Children with Down syndrome (n=60) (μg/g protein) mean ± sem | Siblings without Down syndrome (n=29) (μg/g protein) mean ± sem | t | p value | |
|---|---|---|---|---|
| GSH | 34.02 ± 2.03 | 37.71 ± 3.40 | -0.98 | > .05 |
| GSSG | 2.32 ± 0.17 | 2.74 ± 0.30 | -1.28 | > .05 |
| Total | 36.34 ± 2.03 | 40.45 ± 3.47 | 0.67 | > .05 |
| GSH/Total | 0.93 ± 0.05 | 0.92 ± 0.05 | -9.01 | > .05 |
Red blood cell activity of ACP1, TMR and MHR is presented in Table 2. All enzymatic systems show more enhanced activity in the population without Down syndrome than in the children with Down syndrome, however, there was no statistically significant difference between the two groups.
| Children with Down syndrome | Siblings without Down syndrome | t | p value | |||
|---|---|---|---|---|---|---|
| n | mean ± sem | n | mean ± sem | |||
| ACP1 (μmol/g Hb/min) | 30 | 294.87 ± 31.95 | 16 | 332.93 ± 43.91 | -.70 | > .05 |
| MHR (μmol/g Hb/min) | 42 | 32.90 ± 2.62 | 16 | 34.48 ± 2.95 | -.34 | > .05 |
| TMR (mmol/l cell/h) | 60 | 4.88 ± 0.40 | 27 | 4.98 ± 0.61 | -.23 | > .05 |
Correlation analysis between ACP1 and TMR and MHR activities was performed and Pearson coefficients are presented in Table 3. In our samples the r-values showed weak, non-significant, correlations.
| Children with Down syndrome | Siblings without Down syndrome | |
|---|---|---|
| r | r | |
| ACP1 vs THR | -.16 | -.31 |
| ACP1 vs MHR | -.12 | .11 |
| MHR vs TMR | .24 | .14 |
Correlation analysis between ACP1 and GSH concentrations was performed and the Pearson coefficients are presented in Table 4. The r-value showed a negative and significant correlation, only in the children without Down syndrome, for ACP1 and GSH/GSH+GSSG. None of the other results were significant.
| Children with Down syndrome | Siblings without Down syndrome | |
|---|---|---|
| r | r | |
| ACP1 vs GSH | .10 | -.40 |
Discussion
There was a high level of agreement to take part in the study by the families and the high rate of involvement of siblings demonstrated willingness to contribute to greater knowledge in this area.
TMR, MHR and ACP1 are, as stated above, important during oxidative stress, preventing the formation or converting reactive oxygen species, in order to reduce tissue damage. In the current samples, no difference was found in the two groups between these systems. Thus, in children with Down syndrome no special changes in the anti-oxidant system seem to have been produced to compensate for the higher levels of reactive oxygen species. However, we must consider that this study has some limitations since SOD should have also been measured.
Another limitation is that using siblings as a comparison group, the age difference between the two groups can introduce some bias, and this must be taken into account in interpreting the results.
In children without Down syndrome there is a negative correlation between ACP1 and relative GSH concentrations, since ACP1 modulates glutathione reductase activity. In the group of children with Down syndrome this correlation was not found. This might be associated with a metabolic imbalance, resulting in the accumulation of reactive species, which may be responsible for the premature ageing and tissue damage found in Down syndrome. Further studies will be needed to help clarify these mechanisms.
Correspondence
Mónica Pinto • APPT21, Rua Dr. José Espírito Santo, Lote 49, Loja 1, Chelas, 1900-672 LISBOA, PORTUGAL • Telephone: + 351-218371699 • Fax: + 351-218371712 • E-mail: monicap@mail.pt
Acknowledgements
This article is based on a paper presented at the International Conference on Chromosome 21 and Medical Research on Down Syndrome, Barcelona, 1997. The authors would like to thank Dr Pueschel for reviewing a draft of the article and for his helpful suggestions.
References
- Bar-Peled, O., Korkotian, E., Segal, M. & Groner, Y. (1996). Constitutive overexpression of Cu/Zn dismutase exacerbates kainic acid-induced apoptosis of transgenic-Cu/Zn superoxide dismutase neurons. Proceedings of the National Academy of Sciences of the United States of America, 93(16), 8530-8535.
- Board, P.G. & Pidcock, M.E. (1981). Methemoglobinaemia resulting from heterozygosity for two NADH-Methaemoglobin reductase variants: Characterization as NADH-Ferricyanide reductase. British Journal of Hematology, 47, 361-70.
- Brooksbank, B.W. & Balzas, R. (1983). Superoxide dismutase and lipoperoxidation in Down's syndrome fetal brain. Lancet, 1(29), 881-2.
- Busciglio, J. & Yanker, B.A. (1995). Apoptosis and increased generation of reactive oxygen species in Down syndrome neurones in vitro. Nature, 378, 776-779.
- Carmeliet, G., David, G. & Cassiman, J.J. (1991). Cellular ageing of Alzheimer's disease and Down syndrome cells in culture. Mutation Research, 256(2-6), 221-231.
- Ceballos -Picot, J. (1993). Transgenic mice overexpressing copper-zinc superoxide dismutase as models for the study of free radical metabolism and aging. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales, 187(3), 308-323.
- Chiarugi, P., Cirri, P., Raugei, G., Manao, G., Taddei, L. & Ramponi, G. (1996). Low Mr phosphotyrosine protein phosphatase interacts with the PDGF receptor directly via its catalytic site. Biochemical and Biophysical Research Communications, 219, 21-25.
- Crastes de Paulet, A. (1990). Free radicals and ageing. Annales de Biologie Clinique (Paris), 48(5), 323-330.
- De La Torre, R., Casado, A., López-Fernández, E., Carrascosa, D., Ramirez, V. & Sáez, J.(1996). Overexpression of copper-zinc superoxide dismutase in trisomy 21. Experientia, 52, 871-873.
- De-Haan, J.B., Cristiano, F., Iannello, R., Bladier, C., Kelner, M.J. & Kola, I. (1996). Elevation of the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Human Molecular Genetics, 5(2), 283-292.
- De-Haan, J.B., Cristiano, F., Innello, R.C. & Kola, I. (1995). Cu/Zn-superoxide dismutase and glutathione peroxidase during aging. Biochemistry and Molecular Biology Internals, 35(6), 1281-1297.
- Fuchs, K.R., Shekels, L.L. & Bernlohr, D.A. (1992). Analysis of the ACP1 gene product: classification as an FMN phosphatase. Biochemical and Biophysical Research Communications, 189(3), 1598-1605.
- Gerli, G., Zenoni, L., Locatelli, G.F., Mongiat, R., Piattoni, F., Orsini, G.B., Montagnani, A., Gueli, M.R. & Gualanchi, V. (1990). Erythrocyte antioxidant system in Down syndrome. American Journal of Medical Genetics Supplement, 7, 272-273.
- Hatherill, J.R., Till, G.O. & Ward, P.A. (1991). Mechanisms of oxidant-induced changes in erythrocytes. Agents and Actions, 32(3-4), 351-358.
- Hissin, P.J. & Hilf, R. (1976). A fluorimetric method for determination of oxidized and reduced glutathione in tissues. Analytical Biochemistry, 74(1), 214-226.
- Ishikawa, T. & Sies, H. (1989). Glutathione as an antioxidant: toxicological aspects. In D. Dolphin, R. Paulson & O. Avramovic (Eds.) Glutathione Chemical, Biochemical and Medical Aspects (pp. 85-105). Canada: Wiley Publications.
- Magenis, R.E., Koler, R.D., Lovrien, E., Bigler, R.H., Duval, D.C. & Overton, K.M. (1975). Gene dosage: Evidence for assignment of erythrocyte acid phosphates locus to chromosome 21. Proceedings of the National Academy of Sciences of the United States of America, 72, 4526-4530.
- May, J.M., Qu, Z. & Morrow, J.D. (1996). Interaction of ascorbate and α-tocopherol in resealed human erythrocyte ghosts. The Journal of Biological Chemistry, 271 (18), 10577-10582.
- Orringer, E.P. & Roer, M.E.S. (1979). An ascorbarte mediated transmembrane-reducing system of the human erythrocyte. Journal of Clinical Investigation, 63, 53-58.
- Prasher, V.P. (1993). Down's syndrome, longevity and Alzheimer's disease. British Journal of Psychiatry, 162, 701.
- Pueschel, S.M. (1990). Clinical aspects of Down syndrome from infancy to adulthood. American Journal of Medical Genetics Supplement, 7, 52-56.
- Rubin, L.L., Gatchalian, C.L., Rimon, G. & Brooks, S.F. (1994). The molecular mechanisms of neuronal apoptosis. Current Opinion in Neurobiology, 4(5), 696-702.

